PDF chapter test TRY NOW
In this lesson, we are going to learn about gases and their fundamental laws.
Gas:
Gas is one of the states of matter that has no fixed shape and volume. Gases have a lower density compared to other states of matter, such as solids and liquids.
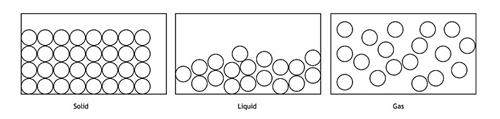
Arrangement of molecules in different states of matter
There is a wide range of space between particles, with a higher kinetic energy than the attractive forces between them. The particles move very fast and interact with others, causing them to diffuse or spread out until they are uniformly distributed throughout the volume of the container.
When more gas particles enter into a container, there is significantly less space for the particles to spread out, and they become compressed.
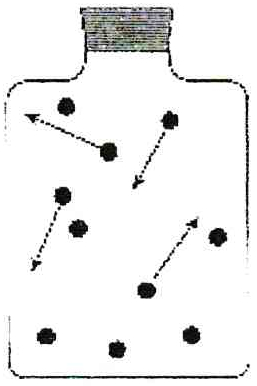
Gas particles in a container
The particles release more force on the interior volume of the container. This force is called pressure. There are severl units used to express the term pressure.
Apart from pressure, denoted in equations as P, gases have other measurable properties such as temperature (T), volume (V) and mole number (n or mol).
Gases have three important characteristic properties:
- Gases are easy to compress,
- Gases expand to fill their containers, and
- Gases occupy far more space than the liquids or solids from which they form.
From the early \(17th century\), the gas laws have been around to help scientists find volumes, pressures, and temperature.
The gas laws consist of three primary laws:
- Charles' law
- Boyle's law and
- Avogadro's law
Reference:
https://commons.wikimedia.org/wiki/File:Solids_liquids_and_gases_-_particle_model.jpg
https://commons.wikimedia.org/wiki/File:Gas_particles_in_a_jar_2.jpg
