
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoThe reaction in which a single compound splits into two or more simpler substances under suitable conditions is called decomposition reaction.
The combination reaction is the exact opposite of this reaction. The following is a general representation of a decomposition reaction:

Decomposition reaction
Breaking bonds is the most important phenomenon in a decomposition reaction; hence, it requires energy to break the bonds.
Depending on the energy source employed in the decomposition reaction, it is classified into three main classes:
(i). Thermal decomposition reactions
(ii). Electrolytic decomposition reactions
(iii). Photo decomposition reactions
Thermal decomposition reactions:
Thermal decomposition reaction is caused by applying heat.
Example: \(1\)
On heating mercury (II) oxide, it is decomposed into mercury metal and oxygen gas as shown below:
As the molecule is dissociated by absorbing heat, it is also called thermolysis. Such reactions, in which heat is absorbed, are called ‘endothermic reactions’. It is a type of compound to element/element decomposition. i.e. a compound (\(HgO\)) is decomposed into two elements (\(Hg\) and \(O\)).
Example: \(2\)
On heating calcium carbonate, it decomposes into calcium oxide and carbon dioxide. It is a type of compound to compound/compound decomposition.
In a thermal decomposition reaction, heat is supplied to break the bonds. Such reactions in which heat is absorbed are called endothermic reactions.
Electrolytic decomposition reactions:
Electrical energy is used in some decomposition reactions to bring the reaction. For example, when an electric current is passed through an aqueous sodium chloride solution, it decomposes into metallic sodium and chlorine gas. This process is called electrolysis.
This is a compound to element/element decomposition as the compound \(NaCl\) is converted into elements \(Na\) and \(Cl\).
Photo decomposition reactions:
Light is another form of energy that helps in decomposition reactions.
Example:
When silver bromide is exposed to light, it decomposes into silver metal and bromine gas.
Since the decomposition is caused by light, this reaction is also called photolysis.
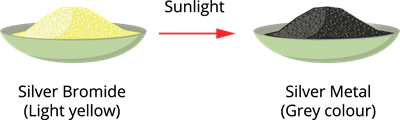
Photodecomposition of silver bromide
It is also a compound toelement/element decomposition.
Differences between combination and decomposition reaction:
Combination reaction | Decomposition reaction |
One or more reactants reacts to form a single product.  | A single reactant is decomposed to give one or more products.  |
| Energy is evolved. | Energy is absorbed. |
| Reactants may elements or compounds. | The reactant is a single compound. |