
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoWe are all aware that many reactions or changes occur every day. However, they are not permanent ones. We can classify them into,
(i) Physical changes
(ii) Chemical changes
Physical change: Physical change is when a substance retains its identity while changing the phase or state without altering its composition. It is temporary and reversible.
For example, liquid water freezes to form ice, which subsequently melts to form liquid water. In other words, freezing is reversed, so it is not a permanent change.
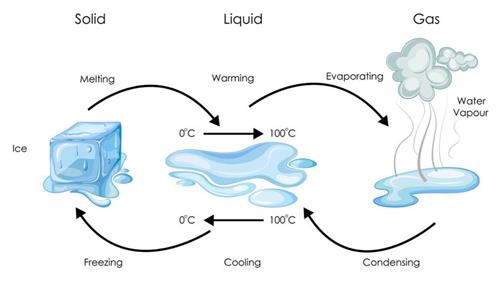
Example for physical change: State change of water
Other examples for physical changes:
Mixing sand with water, crushing a water bottle, boiling water, dissolving sugar or salt in water, breaking glass, chopping wood, etc.
Chemical change:
A chemical change is when a substance loses its identity and forms new products by altering its composition. It is permanent and irreversible.
For example, burning of wood. The carbon compounds in the wood are converted to carbon dioxide gas and water when they are burned.

Burning wood
Other examples for chemical changes: Iron rusting, cooking an egg, baking cake, fireworks, electroplating, etc.
Is it possible to recover the wood from carbon dioxide and water immediately? It is not possible. Hence, it is a permanent change. We cannot do this in most cases; however, some chemical reactions can be reversed.
For example, a charged mobile phone gets energy from its lithium battery by a chemical reaction. The battery of a mobile phone is charged, and the mobile phone receives energy from its lithium-ion battery through a chemical reaction. This process is called discharge. Once the charge is exhausted, we recharge the battery once again. These chemical reactions are reversible.

Charging a mobile phone
Thus, chemical reactions may be reversed under suitable conditions.
Hence, the reactions are grouped into reversible and irreversible reactions.
Reference:
https://cdn.pixabay.com/photo/2016/11/22/14/29/fire-1849385_640.jpg
https://th.bing.com/th/id/OIP.B46PsG2RDfybQEgbLfpTDAHaEP?pid=ImgDet&rs=1