
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoRusting:
Have you ever noticed any reddish-brown flaky substance on the surface of an unused object?
The flaky substance formed on the outer surface of an iron object is known as rust.
Any iron object, when exposed to oxygen in the presence of moisture or any humid environment, leads to the formation of rust. Iron material undergoes rusting when it reacts with oxygen and forms iron oxide.
We can estimate the total percentage of oxygen in the air removed for this rusting process.

Rusting of iron chain
The proportion of oxygen in the air:
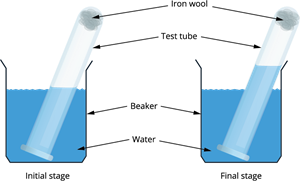
Experimental set-up
- A small portion of iron wool is taken and is pressed into a \(20\ ml\) graduated test tube.
- Now, wet the iron wool with some water and remove the excess.
- A \(500\ ml\) beaker is taken, and it is half-filled with water.
- The test tube is now inverted into the beaker.
- This arrangement is left undisturbed for over a week.
Observation:
An increase in the water level is observed inside the test tube. The water level is raised due to the removal of oxygen in the air by the rusting reaction, which is around \(20\%\). This estimated percentage of oxygen is approximately the total percentage of oxygen in the air.