PDF chapter test TRY NOW
The separation method depends on the size of the particle, shape and physical state (solid, liquid and gas) of the components of the mixture.
There are two different separation methods:
- Separation of solid-liquid mixture
- Separation of solids
Separation of solid-liquid mixture:
This method involves,
- Filtration
- Sedimentation
- Decantation
- Churning
Filtration:
The method of separating insoluble components from a mixture is called filtration.
Example: Removing tea leaves from the liquid

Sedimentation:
The process in which insoluble impurities in the liquid settles down at the bottom of the vessel containing the mixture. The portion that settles down is called sediment.
Example: Separating insoluble mud
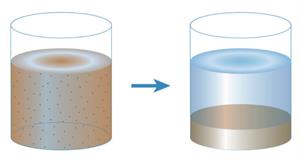
Decantation:
Decantation is the process in which the supernatant liquid that we got from sedimentation is slowly transferred from the container without disturbing the sediment.
Example: Removal of water from the sediment

Churning:
Churning is the process in which very fine insoluble solids are separated from a liquid.
Example: Separating butter from curd

