PDF chapter test TRY NOW
We have studied that all objects are made up of tiny particles called atoms. In this chapter, we will discuss the structure of the atoms in a detailed manner. To understand the concept, let us consider iron rods, bricks and chalk.
When we crush the objects, we can get the powder of it, but when we look at the same powder through a microscope, we can see the molecules of the object. Those molecules are the combination of similar or different atoms. Atom is the basic unit of an element with neutral charge.
Iron rods:
 Iron rod |  Powdered iron |
Bricks:
 Bricks |  Powdered brick |
Chalk:
 Chalk |  Chalk powder |
Let us discuss about size of the smaller objects like the tip of a pin, red blood cell, virus and dust particles to understand the size of an atom.

The size of the pin tip is about .
The size of the red blood cell is about .
The size of the virus is about .
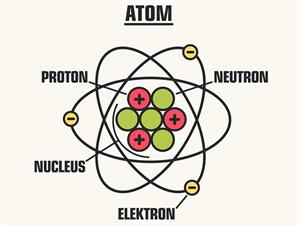
The size of the atom is about.
By comparing all the sizes, atoms are the smallest of all. We cannot see the atoms through our naked eye; then, you can imagine the size of an atom. An atom is a thousand times smaller than the thickest human hair. It has an average diameter of \(0.0000000001\) \(m\) or .
