PDF chapter test TRY NOW
Many scientists gave different theories about the structure of an atom. Let us discuss the theory presented by the following scientists,
- Dalton's atomic theory
- Thomson's theory
- Rutherford's theory
Dalton's atomic theory:

John Dalton
- John Dalton proposed the atomic theory in the year \(1808\).
- He proposed that all the matter was made up of tiny particles called atoms(Indestructible and indivisible building blocks of matter). All the atoms of an element are identical. Different elements have different atomic sizes and mass.
- An atom is the smallest indivisible particle, that is spherical in shape.
- Dalton failed to propose an idea about the charges of an atom(positive and negative charges). Hence, this theory fails to explain many of the properties of substances.
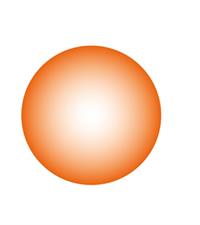
Dalton assumed the shape of the atom is spherical.
Thomson's theory:

J.J Thomson
- In 1897, J.J Thomson proposed a different theory popularly known as 'Plum-pudding model'.
- He compared an atom to a watermelon. The atom has positively charged part like the red part of the watermelon and negatively charged particles, that he called electrons are embedded in it, like watermelon seeds.
- According to this theory, as the positive and negative charges are equal, the atom as a whole does not have any resultant charge. Thomson's greatest contribution was to prove the existence of negatively charged particles or electrons in an atom through experimentation.
- For this discovery, he was awarded the Nobel Prize in \(1906\). Although this theory explained why an atom is neutral, it was an incomplete theory in other ways.
 |  |
J J Thomson's assumption
