PDF chapter test TRY NOW
Melting is an endothermic process. The heat absorbed from the surroundings is used for the reaction to occur, which causes the substance to melt.
The process in which solid changes into a liquid on heating is known as melting.
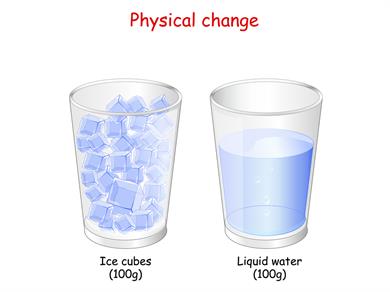
Melting of ice cubes
We know the ice cubes melt when they are kept under heat because they absorb heat from the surrounding air to melt and form water. The appearance of ice and water looks different; they are made up of water molecules.
This refers to no new substance being formed when the ice melts; here, only a state of the substance changes. So, the melting of ice to form water is a physical change.

Melting of ice cream
The ice cream melts when it is kept at room temperature. This is due to the fact that it absorbs heat from the surroundings and changes to the other state with the same chemical composition. This type of change is known as physical change.
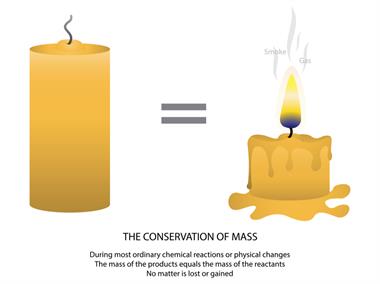
Melting of candle wax
The melting of the candle is also another example of physical change. Here, when the wick is lighted, the candle starts to melt because it absorbs heat from the wick (chemical change), making the candle wax melt (physical change).
Therefore, melting is a physical change that can be reversed. Melting is an example of an endothermic process.
