
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoIndicators are substances that show a colour change when acids or alkalis are added to them. They are,
- Natural indicators
- Synthetic indicators
Natural indicators
These are chemical compounds that come from natural sources.
Litmus, turmeric juice, China rose petals, red cabbage, grape juice and beetroot juice are examples of natural indicators. Let see about them in detail.
Litmus:
The most common indicator is litmus, which is derived from lichens. It comes as a solution or strips made by absorbing litmus solution onto filter paper. We mostly use it in the form of litmus paper, which comes in two colours: blue and red.
Acids convert blue litmus into red, while base or alkali converts red litmus into blue, as shown below:
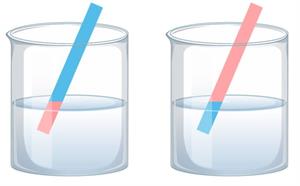
Test for acid and base using litmus papers
When a red litmus paper is immersed in an acidic solution, it has no effect. Similarly, as shown below, when a blue litmus paper is immersed in a basic solution, it has no effect.
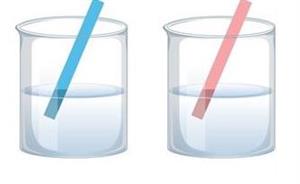
No effects on the litmus
When litmus papers are used to test a neutral solution, the same result (see the above picture) occurs.
Neutral solutions are those in which the concentrations of hydrogen and hydroxide ions are equal. Examples, sodium chloride solution, sugar solutions and water.
Turmeric indicator:
How to make a turmeric indicator and make use of it?
A paste is made by mixing turmeric powder with a small amount of water. This is then dried on blotting paper or filter paper. These strips are used as indicators to determine the solution's nature. Turmeric indicator paper does not change colour in an acidic solution; it remains yellow. In the basic solution, the colour changes from yellow to red.

Turmeric indicator
Hibiscus flower indicator:
This indicator is made by soaking some hibiscus petals in warm water for \(5\) to \(10\) minutes. After that the solution can be used as an indicator. In an acidic solution, the colour changes to deep pink or deep red. In the basic solution, the colour
changes to green.
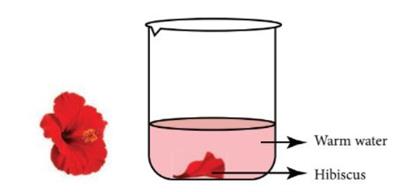
Hibiscus solutions as indicator
Image credits: (Acids and Bases_8 TN board_Chapter 5, page no. 62)
Red cabbage:
How to make a red cabbage indicator and make use of it?
Red cabbage should be cut into small pieces. Place these pieces (minimal amount) in a bowl of hot water for a few minutes. Allow them to stand for a while until the water turns purple in colour. Take the filtrate from the filter after the watercolour has changed, and allow it to cool slowly. Take the filter paper and dip it into the filtrate. Take it out and let it dry completely once it has been soaked. We can cut the filter paper into strips, similar to pH paper strips, if necessary.


Cabbage indicator