PDF chapter test TRY NOW
Do you know?
Anhydrous copper (II) sulphate powder is white in colour. It becomes blue when you add water to it, as shown below.
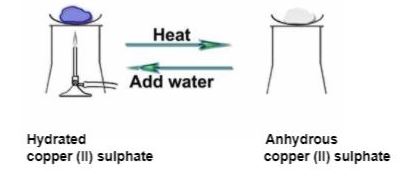
Colour changes on the substance when adding water
This is one of the tests to determine the presence of water.
Preparation of water
In \(1781\), Henry Cavendish, an English scientist, was the first person to prepare water. While performing this experiment, he discovered hydrogen gas when active metals reacted with sulphuric acid. The hydrogen gas released was highly flammable, and when it was burned, it turned into a colourless substance known as water. He also made carbon dioxide by mixing metals with strong bases.
The following activity provides water:
- Reduction of metal oxide by hydrogen.
- Combustion of hydrogen in the air.
- Burning of hydrocarbons in the air.
- Plants and animals both release water through their respiration.
How to prepare water in a laboratory?
This process involves passing pure hydrogen gas through anhydrous calcium chloride to absorb any water vapour that may be present. Then, with enough air, the dry hydrogen that comes out of the aperture is burned. When the burning hydrogen gas comes into touch with the cold flask, it generates water droplets. This procedure produces distilled water that is free of dissolved particles.
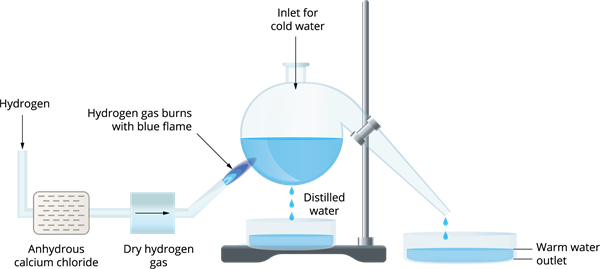
Preparation of water
