PDF chapter test TRY NOW
When you say salt, you may think of the white stuff we use when cooking, but that is one kind of salt called common salt or sodium chloride (\(NaCl\)). Seawater contains many salts dissolved in it. Sodium chloride is an exception to these salts.
There are different salts used in various fields. The reaction between an acid and a base forms a neutral product called salt. When salts are dissolved in water, they produce both positive and negative ions.
Types of salts:
- Normal salt
- Acid salt
- Basic salt
- Double salt
Normal salt: Normal salt is formed when a base completely neutralizes an acid.
\(NaOH + HCl → NaCl + H_2O\)
Acid salt: It is produced when a metal partially replaces the hydrogen ions in an acid. Acid salt is formed when a calculated amount of a base is added to a polybasic acid.
\(NaOH + H_2SO_4 → NaHSO_4+ H_2O\)
Basic salt: Basic salts are formed when the hydroxide ions of a diacidic or triacidic base are partially replaced with an acid radical.
\(Pb(OH)_2+ HCl → Pb(OH)Cl + H_2O\)
Double salt: Double salts are formed by blending saturated solutions of two simple salts in an equimolar ratio and then crystallising them. For example, potash alum is a combination of potassium sulphate and aluminium sulphate.
Properties of salts:
- The salts are often crystalline solids with a salty taste.
- The majority of salts are water-soluble.
\(KCl\) and \(NaCl\) are two examples of soluble salts. \(AgCl\) is an example of an insoluble salt. - The melting and boiling points of solid salts are very high.
- Salts produce electricity when they are molten or liquid, but not when they are solid.
Water of Crystallisation
Many salts are found as crystals containing water molecules. These water molecules are called "water of crystallisation". The salts that contain water of crystallisation are known as hydrated salts.
The number of molecules of water hydrated in salt is mentioned after a middot in its chemical formula.
Example:
The formula for copper sulphate pentahydrate is . The number "\(5\)" indicates that the crystal has five water molecules for each molecule of copper sulphate. Copper sulphate is blue in appearance due to this water of crystallisation. It loses its water molecules and becomes white when heated.
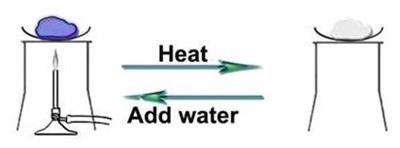
The appearance of copper sulphate when heating and adding water
The salts that do not have water of crystallisation are called anhydrous salt. They are generally found as powders.
Reference:
https://th.bing.com/th/id/OIP.OrJHh1TnLsD-n4Q4QR2rkwHaDt?pid=ImgDet&rs=1
