PDF chapter test TRY NOW
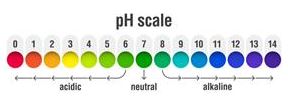
pH refers to the potential of hydrogen or the power of hydrogen. The pH scale ranges from \(0\) to \(14\), which is used to indicate whether a solution is acidic, basic or neutral.
pH scale
The pH of acids, base and neutral solution are listed below:
Acid: pH < \(7\)
Base: pH > \(7\)
Neutral: pH = \(7\)
What is the best way to determine the pH of a solution?
A solution's pH can be measured using a universal indicator. It is made up of a number of different colours. It is available as a solution or as a piece of pH paper.
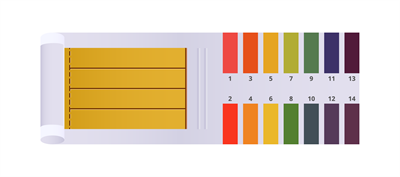
Universal indicator as pH paper

Universal indicator as solutions
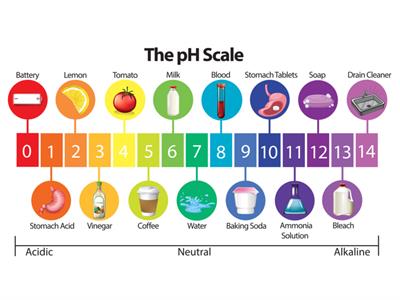
pH of some things around us:
pH scale
Importance of pH in daily life
Our bodies work in a pH range of \(7.0\) to \(7.8\). Only a small range of pH change allows living organisms to live.
pH in our digestive system:
Our stomachs contain hydrochloric acid, which helps in food digestion, thus causing no harm to the stomach. The stomach produces too much acid during indigestion, causing pain and irritation. The pH of stomach fluid is about \(2.0\).
pH of soil:
The pH of the soil is important in agriculture. For example, citrus fruits require slightly alkaline soil, while rice and sugarcane require acidic soil and neutral soil, respectively.
pH of rainwater:
The pH of rainwater is approximately \(7\), which indicates it is neutral. If the atmospheric air is polluted with sulphur and nitrogen oxides, they get dissolved in rainwater and make the rainwater's pH less than \(7\). Acid rain is described as rainwater with a pH of less than \(7\). Acid rain decreases the pH of river water as it falls into them. In such rivers, aquatic life has a hard time living.
Some solution's pH are listed below:
Solutions | pH range |
| Blood | \(7.3\) - \(7.5\) |
| Saliva | \(6.5\) - \(7.5\) |
| Gastric juice | \(1.0\) - \(3.0\) |
| Rainwater | \(7\) |
| Seawater | \(8.5\) |
| Tomato juice | \(4.0\) - \(4.4\) |
