PDF chapter test TRY NOW
Electrochemistry:
We use many electronic devices like mobile phones and electrical appliances like a torchlight in our daily lives. Electricity produced by the battery is the crucial factor that makes these devices function.
But how does the battery produce electricity?
Because it contains some chemicals in it. The chemical reactions (chemical energy) in the battery produce electricity (electrical power).
Electrochemistry is another branch of Applied chemistry where scientists convert chemical energy into electrical energy and vice versa.

Cell or battery
Electrochemistry is a part of chemistry that deals with the relation between electrical energy and chemical change. It is mainly concerned with the processes between the electrode and solution having ions called an electrolyte.
Electrochemical Cell:
A variety of chemical reactions take place around us. Do all produce electricity?
No.
Only redox reactions that take place in a specific device can produce electricity. An electrochemical cell is a device that uses a chemical change to produce electricity or electricity to produce a chemical change.

(a). Components of Electrochemical Cell:
An electrochemical cell may contain the following two major segments.
Electrode: It is a solid electrical conductor made of metal (sometimes non-metal like graphite). A cell consists of two electrodes. One is called the anode, and another one is a cathode.
Electrolyte: This is a substance made up of ions or molten salts that conduct electricity.
Electrolyte: This is a substance made up of ions or molten salts that conduct electricity.
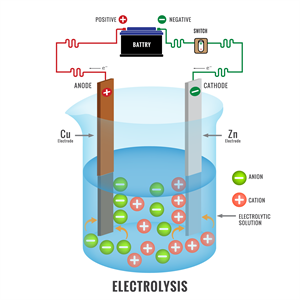
(b). Cell reactions: An electrochemical cell involves two reactions together.
Oxidation: As we already know, oxidation is the loss of an electron. The anode of electrochemical cells undergoes oxidation.
Metal → Metal ion + electron (e-)
Reduction: It involves the gain of an electron. Reduction occurs at the cathode.
Reduction: It involves the gain of an electron. Reduction occurs at the cathode.
Metal ion + electron (e-) → Metal
Since both reactions occur together, the interconversion of electrical and chemical energy in electrochemical cells involves a redox reaction.
