PDF chapter test TRY NOW
(c). Electrochemical Cell Types:
Based on the nature of the energy transformation, electrochemical cells are broadly divided as below.
Galvanic Cell:
An electrochemical cell converts chemical energy into electrical energy or creates electricity from chemical reactions.
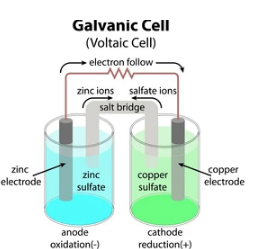
- Galvanic cell is made up of two half cells, one anodic and the other cathodic.
- The anodic half-cell anode contacts the electrolyte, whereas, in a cathodic half-cell, the cathode connects with its electrolyte.
- A conductor wire connects the anode and cathode.
- The electrolytes of half-cells are joined through a tube carrying a saturated salt solution. It is called a salt bridge.
- Thus, in a galvanic cell, both the half-cells are kept individually but stay connected electrically.
How does a Galvanic cell produce Electricity?
At the anode, oxidation occurs, releasing electrons. The cathode attracts these electrons, and hence the electrons flowing from anode to cathode are gained in the reduction. Thus, the redox reaction yields the flow of electrons and electricity.
Electrolytic Cell:
An electrochemical cell that converts electrical energy to chemical energy, i.e., electricity is used to initiate chemical reaction in electrolytic cells.
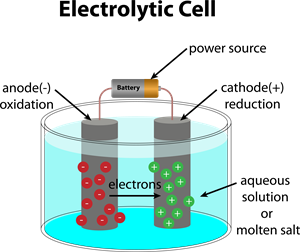
We get electricity from Galvanic cells. But electrolytic cells use electricity. So then how are they useful?.
In electrolytic cells, when electricity is given to the electrolyte, it dissociates into its component ions. Then, these ions undergo a redox reaction creating the individual elements. This phenomenon is termed as Electrolysis. So, Electrolysis is a method by which an electrolyte is decomposed into its part elements by moving electricity into its aqueous solution or fused (molten) state.
(b). Significance of Electrochemistry:
The subject of electrochemistry is of great importance. Some of its applications are given below.
- Electrochemistry has been used to discover critical technical processes for producing and purifying non-ferrous metals and the electro-synthesis of organic compounds.
- It is used to determine whether a selective reaction will happen or not.
- The electrochemical redox reaction of ethanol can be used to identify alcohol in drunk drivers.
- Electrochemical reactions are used in the extraction of metals like Aluminium and Titanium from their ores.
- Lithium-ion batteries, lead-acid batteries and fuel cells are based on electrochemical cells. The fuel cell is used in the direct conversion of chemical energy into electrical energy.
