
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoElectric charge is one of the basic fundamental properties of all matter, like mass and volume. Coulomb is the unit of measurement for electric charge, and it is denoted as '\(C\)'.
The charge of an electron (\(e\)) has a very small numerical value. The fundamental unit of electron charge (\(e\)) is equal to \(1.6\ C\). It means that any charge (\(q\)) must be an integral multiple (\(n\)) of the fundamental unit of electron charge (\(e\)).
\(q = ne\)
Where \(q\) is the charge, \(n\) is a whole number, and \(e\) is the charge of an electron.
Calculate the number of electrons present in one coulomb of charge.
Charge of \(1\) electron,
The formula is,
(or)
Number of electrons in \(1\ coulomb\),
Other units of electric charge:
Some other units of electric charge are \(micro\ coulomb\) (\(µC\)), \(nano\ coulomb\) (\(nC\)) and \(pico\ coulomb\) (\(pC\)). The values of the units in terms of a coulomb is given as,
Some other units of electric charge are \(micro\ coulomb\) (\(µC\)), \(nano\ coulomb\) (\(nC\)) and \(pico\ coulomb\) (\(pC\)). The values of the units in terms of a coulomb is given as,
\(1\ µC\) \(=\) \(10^{-6}\ C\)
\(1\ nC\) \(=\) \(10^{-9}\ C\)
\(1\ pC\) \(=\) \(10^{-12}\ C\)
Property of electric charge:
Important!
Electric charge is additive in nature.
The total electric charge of a system is the algebraic sum of all the charges located in the system.
Imagine a system having two charges \(+\ 6C\) and \(–\ 3C\), respectively. Then, the total charge of the system is
\((+6C)\) \(+\) \((–3C)\) \(=\) \(+3C\)
Newton's third law governs the electrostatic forces between two point charges. The action force is on one charge, and the reaction force is on the other, and vice versa.
Electric force:
There are two forms of electric force (\(F\)) among electric charges: attractive force and repulsive force. Charges that are similar repel each other, while those that are unlike attract each other. The 'electric force' is the force that exists between the charges. These forces can be felt even if the charges are not in contact.
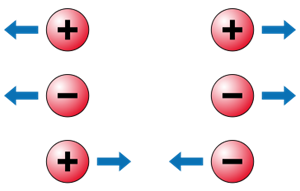
Electric force between charges
Important!
Like charges repel and unlike charges attract each other.
Reference:
https://upload.wikimedia.org/wikipedia/commons/thumb/d/de/Charges_repulsion_attraction.svg/364px-Charges_repulsion_attraction.svg.png