
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoA layer of air surrounds nearly \(300\) \(km\) from the earth surface up to a certain height, and this layer of air around the earth is called an atmosphere. Air exerts pressure because it occupies space and has a weight. We call it Atmospheric pressure. The air pressure at sea level is what we usually mean by atmospheric pressure.
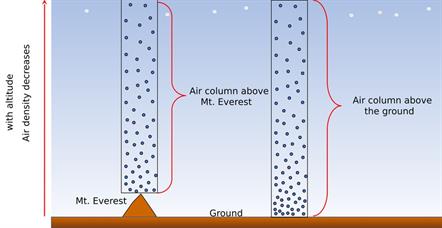
The pressure variation near the ground level and above the ground level is depicted in the below diagram. Since the particles are closer to the ground, the pressure at the ground level will be higher, and the pressure above the ground level will be lower.
Variation of pressure
The air gets thinner when we move to a higher altitude. Therefore, the atmospheric pressure decreases as we go up in the mountains. But, on the other hand, the air gets heavier as we go down below sea level, like mines.
The human lungs are well adapted to breathe (\(101.3\) \(kPa\)) at sea level. Mountain climbers require specialised breathing equipment with oxygen cylinders as pressure drops at higher altitudes. People who work in mines with pressures higher than sea level use similar specialised equipment.

Mountain climbers
Measurement of atmospheric pressure:
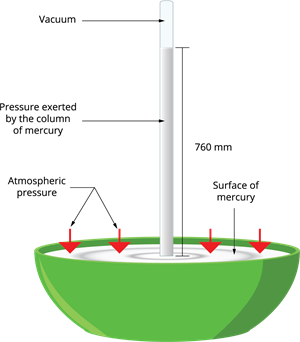
We use the instrument called 'barometer' to measure the atmospheric pressure. The first barometer was designed by an Italian Physicist Torricelli, consists of a long glass tube that is closed at one end, open at the other end. The tube is loaded with mercury and turned upside down into a vessel of mercury. This is done by closing the open end of the mercury-filled tube with the thumb and then opening it after immersing it into a trough of mercury.
We use the instrument called 'barometer' to measure the atmospheric pressure. The first barometer was designed by an Italian Physicist Torricelli, consists of a long glass tube that is closed at one end, open at the other end. The tube is loaded with mercury and turned upside down into a vessel of mercury. This is done by closing the open end of the mercury-filled tube with the thumb and then opening it after immersing it into a trough of mercury.
Barometer
The barometer works by adjusting the mercury level in the glass tube against the outside air pressure. If the pressure increases, mercury rushes into the tube, and if the air pressure decreases, the mercury level declines from the tube. There is no air present in the space between mercury and the closed-end; there is a vacuum in that space. We learnt already that vacuum does not exert any pressure. So the level of mercury present in the tube gives an exact measure of air pressure called atmospheric pressure.
The height of the mercury column at sea level is \(760\) \(mm\).
The density of mercury is \(13600\) . Let us compute the pressure due to the mercury column of \(760\) \(mm\), which is equal to the atmospheric pressure.
The above value is called one atmospheric pressure and is denoted by \(atm\). Another unit called \(bar\) is also used to express such high-pressure values.
Reference:
https://www.piqsels.com/en/public-domain-photo-jfcje