
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoThe term ‘latent' refers to something that is hidden. As a result, latent heat is defined as 'hidden heat' or 'hidden energy'.
Let us take a look at the activity below to better understand latent heat.
- Put some crushed ice cubes in a beaker and use a thermometer to record the temperature. It will be \(0\) degrees Celsius.
- Heat the ice cubes in the beaker.
- Ice is melting to form water, as you can see. Keep track of the temperature at regular intervals; it will remain at \(0\) degrees Celsius until all of the ice has been converted to liquid.
- Repeat the beaker and take another temperature reading.
- You will notice that the temperature rises to \(1000 C\) and stays there even after continuing to heat until the entire mass of water in the beaker is vaporized.
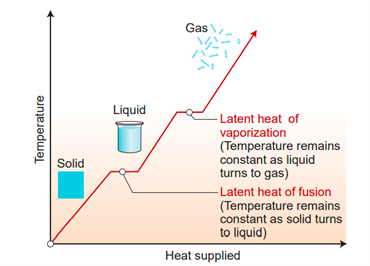
Latent heat
The temperature in the above activity is kept constant at \(0°C\) until all of the ice has been converted to liquid, then it is kept constant at \(100°C\) until all of the ice has been converted to vapour.
Why?
It's because when a substance transforms from one state to another, it absorbs or releases a significant amount of heat energy. Latent heat is the name given to this energy. As a result, latent heat is the amount of heat energy absorbed or released by a substance during a change in its physical states that occurs without a change in temperature.
The solid absorbs heat energy during melting, and the liquid releases an equal amount of heat energy during freezing, with no temperature change. The latent heat of fusion is what it's called. A liquid absorbs heat energy during vaporization, and the vapour releases an equal amount of heat energy during condensation, with no temperature changes.