
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoAll the substances in our surroundings are made up of atoms and molecules. Generally, these molecules are in motion and possess kinetic energy. At the same time, each molecule exerts an attractive force on other molecules, and so they possess potential energy.
Heat energy:
The internal energy of the molecules is the sum of kinetic and potential energy. When this internal energy flows out, it is referred to as heat energy.
This energy is more concentrated in hot substances and less concentrated in cold substances, and it flows from hot to cold. You will learn how this heat transfer occurs in this lesson. You will also review the effects of heat, heat capacity, state change, and latent heat.
Attractive force between the molecules
Which object has more energy in it? Is it a hot object or a cold object?
Things that are hotter have more heat energy than things that are colder. This is because atoms or molecules in hot things move around faster than they do in cold things. The kinetic theory is the name for this concept.
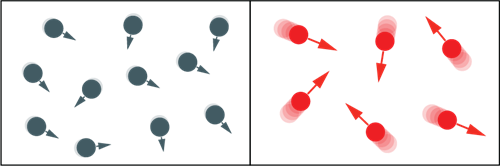
Movement of particles when the object is hot and cold
According to the kinetic theory of matter, all matter is made up of a large number of very small particles that are constantly moving. The amount of energy and the particle's relationship to other particles determine how much they move.
Reference:
https://pixabay.com/illustrations/abstract-molecules-science-atoms-5979469/