PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoHave you ever wondered why ice turns into puddles of water on a sweltering day?
We already learn that matter occurs in three states - solid, liquid and gas.
Knowing that matters can change its states; when a state change occurs, the properties of substances also change. Similarly, if the state change is reversed, the substance will recover the properties which it had before.
Let us do the following experiments to understand this concept clearly.
Experiment 1:
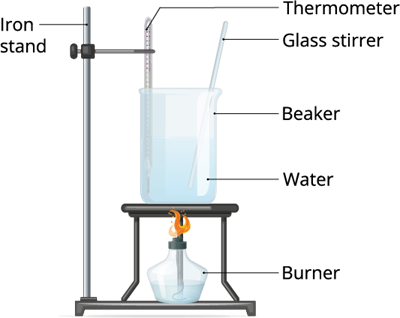
Take some water in a vessel and heat it in medium flame. After a couple minutes observe what happens.
Experiment 2:
Take some ice pellets and keep it in room temperature or expose it in a low flame. Observe what happens to those ice pellets after a couple of minutes.
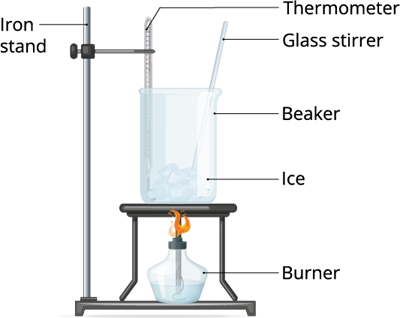
In the above two experiments, we treated the liquid and the solid (ice) with heat. And, we can observe that both liquid and the solid changes its state.
Therefore, when we apply heat energy to the liquid, it becomes gas. And, when we do the same thing to the ice, it becomes liquid. Therefore, we can define the above process as Vaporization and Condensation.
Vaporization: The process where liquid converts its physical state to gaseous state is known as vaporization.
Condensation: The process of the gas converting its physical state to liquid state is known as Condensation. This is also called as reverse vaporization.
But, the question is how this transformation happen?
How matters transforms its states?
When we apply the heat energy to the solid, the particles in the solids absorb the heat energy, and this additional energy breaks the attracting force between the molecules.
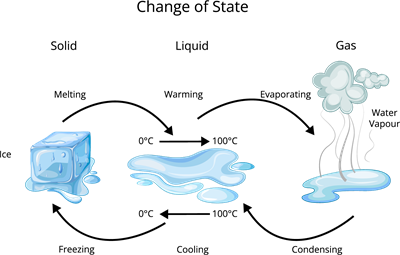
Transformations of states
This leads to the rearrangement to the substance because the attracting force no longer holds the particles tightly. That's why the solid ice cube melts and transform to liquid when heat is applied.
This same will happen if liquid heated, the attractive forces between the molecules break, which leading them to widely dispersed in order a gas to form.
Boiling Point:
The temperature at which a liquid begins to boil at the atmospheric pressure is known as its boiling point.
Similarly, when the solid objects are heated, the attractive force between the molecules breaks, then the particles leave their fixed positions and start moving more freely.
Melting Point:
The temperature at which a solid substance melts to become a liquid at the atmospheric pressure is called the melting point of that substance.
Therefore, the matter changes its state respective to the energy applied to it. The following figure explains how the matter changes its state wherever the energy is applied.